IPSC-derived NK Cells Clinical Trial Pipeline Gains Momentum: 12+ Companies Lead the Charge in Pioneering New Treatments | DelveInsight
The iPSC-derived NK cells clinical trial analysis report delivers important insights into ongoing research of 15+ pipeline iPSC-derived NK cells drugs, clinical strategies, upcoming therapeutics, and commercial analysis.
New York, USA, Feb. 16, 2026 (GLOBE NEWSWIRE) -- IPSC-derived NK Cells Clinical Trial Pipeline Gains Momentum: 12+ Companies Lead the Charge in Pioneering New Treatments | DelveInsight
The iPSC-derived NK cells clinical trial analysis report delivers important insights into ongoing research of 15+ pipeline iPSC-derived NK cells drugs, clinical strategies, upcoming therapeutics, and commercial analysis.
DelveInsight’s 'IPSC-derived NK Cells Pipeline Insight 2026' report provides comprehensive global coverage of pipeline therapies for IPSC-derived NK cells across various stages of clinical development. The report offers an in-depth analysis of key trends, emerging therapies, and competitive landscape dynamics, highlighting the strategies of major pharmaceutical companies to advance the pipeline and capitalize on future growth opportunities. In addition, it includes critical insights into clinical trial benchmarking, partnering and licensing activities, and regulatory pathways involving the FDA and EMA, enabling stakeholders to make informed decisions and optimize development strategies within the IPSC-derived NK cells domain.
IPSC-derived NK Cells Clinical Trial Analysis Summary
- DelveInsight’s IPSC-derived NK cells pipeline report depicts a robust space with 12+ active players working to develop 15+ pipeline IPSC-derived NK cells drugs.
- Key IPSC-derived NK cells companies, such as Centuary Therapeutics (NASDAQ: IPSC), Cartherics Pty Ltd, Fate Therapeutics (NASDAQ: FATE), Nuwacell Biotechnologies Co., Ltd., HebeCell, Healios (TYO: 4593), and others are evaluating new IPSC-derived NK cells drugs to improve the treatment landscape.
- Promising pipeline IPSC-derived NK cells therapies, such as CNTY-101, CTH-401, FT522, NCR300, HC101a, AKT-01, and others, are in different phases of IPSC-derived NK cells clinical trials.
- Approximately 3+ IPSC-derived NK cell drugs are in the early stages of development.
Request a sample and discover the recent advances in IPSC-derived NK cells drugs @ https://www.delveinsight.com/sample-request/ipsc-derived-nk-cells-pipeline-insight?utm_source=globenewswire&utm_medium=pressrelease&utm_campaign=spr
What are IPSC-derived NK Cells?
Induced pluripotent stem cell (iPSC)–derived natural killer (NK) cells are immune effector cells generated by differentiating reprogrammed human somatic cells into NK cells under controlled laboratory conditions. These cells retain the innate cytotoxic properties of NK cells, including the ability to recognize and kill virus-infected or malignant cells without prior antigen sensitization. iPSC-derived NK cells offer key advantages over donor-derived NK cells, such as a renewable, standardized, and scalable “off-the-shelf” source with consistent phenotype and function. They can also be genetically engineered at the iPSC stage to enhance tumor targeting, persistence, or resistance to immunosuppressive tumor microenvironments, making them a promising platform for next-generation cancer immunotherapies.
Find out more about IPSC-derived NK cells drugs @ IPSC-derived NK Cells Treatment
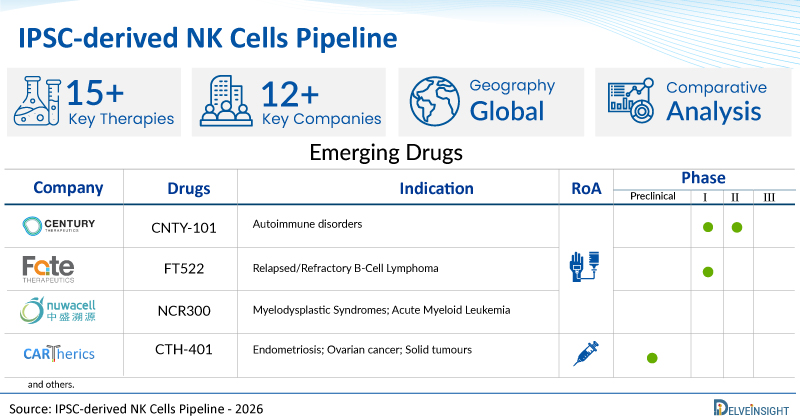
A snapshot of the Pipeline IPSC-derived NK Cells Drugs mentioned in the report:
| Drugs | Company | Phase | Indication | RoA |
| CNTY-101 | Century Therapeutics | I/II | Autoimmune disorders | Intravenous |
| FT522 | Fate Therapeutics | I | Relapsed/Refractory B-Cell Lymphoma | Intravenous |
| NCR300 | Nuwacell Biotechnologies Co., Ltd. | I | Myelodysplastic Syndromes; Acute Myeloid Leukemia | Intravenous |
| CTH-401 | Cartherics Pty Ltd | Preclinical | Endometriosis; Ovarian cancer; Solid tumours | Intraperitoneal |
| HC101a | HebeCell | Preclinical | Pulmonary metastasis of Pediatric Sarcoma | NA |
Learn more about the emerging IPSC-derived NK cells therapies @ IPSC-derived NK Cells Clinical Trials
Stuti Mahajan, consulting manager at DelveInsight, said iPSC-derived NK cells are poised to become a cornerstone of next-generation immunotherapy by overcoming key limitations of primary NK cells, including donor variability, limited scalability, and functional heterogeneity. Their clonal uniformity, inexhaustible supply, off-the-shelf manufacturability, and genetic engineering flexibility drive strong market momentum. With advancing clinical validation and maturing manufacturing and regulatory frameworks, iPSC-NK cells are expected to evolve from a promising innovation into a foundational, standardized, and broadly accessible modality in cancer immunotherapy.
Recent Developments in IPSC-derived NK Cells Treatment Space
- In November 2025, Century Therapeutics announced that CNTY-101 clinical development activities will continue in CARAMEL, a Phase I/II investigator-sponsored trial (IST) led by Professors Georg Schett and Andreas Mackensen and sponsored by the Friedrich-Alexander University Erlangen-Nürnberg. As of November 12, 2025, three B-cell-mediated autoimmune disease patients were treated in this IST. Initial clinical data from CARAMEL is expected to be presented by the trial investigators at the 14th Annual BMT & Cell Therapy Workshop on December 5, 2025. As part of the company’s clinical development re-prioritization efforts, Century will be discontinuing its company-sponsored CALiPSO-1 trial in which five patients have been treated with a favorable safety profile with no DLTs, no CRS >grade 2, and no ICANS. In addition, the limited but emerging clinical data suggest encouraging clinical activity in refractory patient populations.
- In October 2025, ViGenCell signed a joint development agreement with Therabest for TB-420, an iPSC-derived NK cell therapy expressing GPC3 CAR to treat hepatocellular carcinoma. Under this agreement, ViGenCell will pay Therabest a contract fee of 4 billion won (USD 2.8 million), along with milestone payments based on development stages. Revenue will be shared according to a mutually agreed ratio upon technology transfer and upon the generation of independent sales.
- In November 2024, Fate Therapeutics, Inc. presented initial clinical and translational data from the Company’s Phase I study of FT522 in relapsed / refractory B-cell lymphoma at the American College of Rheumatology (ACR) Convergence being held in Washington, D.C. FT522 is the Company’s off-the-shelf, CD19-targeted chimeric antigen receptor (CAR) natural killer (NK) cell product candidate that incorporates multiple novel synthetic controls of cell function designed to target and deplete pathogenic immune cells, and is the Company’s first product candidate to integrate its alloimmune defense receptor (ADR) technology to enable effective treatment of patients without administration of intense conditioning chemotherapy.
- In February 2024, Cartherics Pty Ltd announced that it had completed a pre-investigational new drug (pre-IND) meeting with the US Food and Drug Administration (FDA) for a Phase I/II clinical trial of its lead “off-the-shelf” natural killer (NK) cell therapy product, CTH-401, for the treatment of ovarian cancer.
- In October 2020, Ankarys Therapeutics Inc., Applied StemCell, Inc. (ASC), and HebeCell Corp. (HebeCell) announced that they had entered into a strategic collaboration to co-develop allogeneic induced pluripotent stem cell (iPSC)-derived chimeric antigen receptor (CAR) NK cell therapeutics targeting hematological malignancies. The collaboration will leverage Ankarys’ cGMP iPSC lines, proprietary CAR constructs, and FailSafe cell engineering technology. Ankarys will lead the Canadian CTA/US IND-enabling studies and launch a Phase I clinical trial in Canada and the US.
Scope of the IPSC-derived NK Cells Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Key IPSC-derived NK Cells Companies: Centuary Therapeutics (NASDAQ: IPSC), Cartherics Pty Ltd, Fate Therapeutics (NASDAQ: FATE), Nuwacell Biotechnologies Co., Ltd., HebeCell, Healios (TYO: 4593), and others.
- Key IPSC-derived NK Cells Pipeline Therapies: CNTY-101, CTH-401, FT522, NCR300, HC101a, AKT-01, and others.
Dive deep into rich insights for new IPSC-derived NK cells treatments, visit @ IPSC-derived NK Cells Drugs
Table of Contents
| 1. | IPSC-derived NK Cells Pipeline Report Introduction |
| 2. | IPSC-derived NK Cells Pipeline Report Executive Summary |
| 3. | IPSC-derived NK Cells Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | IPSC-derived NK Cells Clinical Trial Therapeutics |
| 6. | IPSC-derived NK Cells Pipeline: Late-Stage Products (Pre-registration) |
| 7. | IPSC-derived NK Cells Pipeline: Late-Stage Products (Phase III) |
| 8. | IPSC-derived NK Cells Pipeline: Mid-Stage Products (Phase II) |
| 9. | IPSC-derived NK Cells Pipeline: Early-Stage Products (Phase I) |
| 10. | IPSC-derived NK Cells Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the IPSC-derived NK Cells Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the IPSC-derived NK Cells Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the IPSC-derived NK cells cure research, reach out @ Medication for IPSC-derived NK Cells Treatment
Related Reports
NK Cell Therapy Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key NK cell therapy companies, including Artiva Biotherapeutics, Glycostem, Fate Therapeutics, Indapta Therapeutics, Senti Biosciences, among others.
NK Cell Therapy Clinical Trial Analysis
NK Cell Therapy Pipeline Insight – 2026 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key NK cell therapy companies, including Amgen , Innate Pharma , Nektar Therapeutics , SMT bio Co., Ltd., Alphageneron Pharmaceuticals, XNK Therapeutics, ImmunityBio, Cellid, Cantargia, Affimed Therapeutics, Takeda, Artiva Biotherapeutics, Sanofi, Dragonfly Therapeutics, INmune Bio, NKGen Biotech, Asclepius Technology Company Group, Glycostem Therapeutics (IPD Therapeutic), Wugen, Celularity, VERAXA, GamidaCell, MiNK Therapeutics, Indapta Therapeutics, ImmunityBio, Inc., Allife Medical Science and Technology, Nkarta, Base Therapeutics, GT Biopharma, Athenex, Ambicion, Biohaven Pharmaceuticals, Acepodia, Bright Path Biotherapeutics, Nkarta Therapeutics, Qihan Biotech, Century Therapeutics, Fate Therapeutics, Chimeric Therapeutics, Senti Biosciences, GICELL, Deverra Therapeutics, Medigen Biotechnology Corporation, GlaxoSmithKline , CytoImmune Therapeutics, Nuwacell Biotechnologies Co., Ltd., among others.
Rheumatoid Arthritis Clinical Trial Analysis
Rheumatoid Arthritis Pipeline Insight – 2026 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key rheumatoid arthritis companies, including Gedeon Richter, mAbxience Research S.L., AnaptysBio, Inc., Istesso, Synact Pharma, Sanofi, AstraZeneca, Cullinan Therapeutics, Sonoma Biotherapeutics, Immunovant, Inc., Janssen Research & Development, LLC, Ernexa Therapeutics Inc., Kymera Therapeutics, Inc., Eli Lilly and Company, Rise Therapeutics LLC, Artiva Biotherapeutics, Inc., SinoMab BioScience Limited., ECM Therapeutics, Hangzhou Highlightll Pharmaceutical Co., Ltd, Beijing VDJBio Co., LTD., AbbVie, ILAb Co., Ltd., Sonoma Biotherapeutics, Inc., Gilead Sciences , Zenas BioPharma (USA), LLC, Flerie AB, Spyre Therapeutics, Inc., Candid Therapeutics, Inc., LAPIX Therapeutics, among others.
Ovarian Cancer Clinical Trial Analysis
Ovarian Cancer Pipeline Insight – 2026 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key ovarian cancer companies, including Allarity Therapeutics, OSE Immunotherapeutic, Cristal Therapeutics, Bristol-Myers Squibb , Ono Pharmaceuticals, Merck KGaA & Co., Aravive Biologics, Mersana Therapeutics, Clovis Oncology, Verastem Oncology, Gradalis, AbbVie, Elevation Oncology, OncoQuest Pharmaceuticals (CanariaBio), Alkermes , Hoffman-La Roche , AstraZeneca, MSD, GlaxoSmithKline , IMV, Corcept Therapeutics, among others.
Endometriosis Clinical Trial Analysis
Endometriosis Pipeline Insight – 2026 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key endometriosis companies, including Gedeon Richter, Changchun GeneScience Pharmaceutical Co., Ltd., Jiangsu Hengrui Medicine Co., Ferring Pharmaceuticals, Chugai Pharmaceutical, Foraviset, EpicentRx, NETRIS Pharma, Maipl Therapeutics, Flightpath Biosciences, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com


