BTK Inhibitors Clinical Trial Pipeline Accelerates as 30+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
Overall, BTK inhibitors and ICH-related therapies sit in a space with strong scientific momentum but notable hurdles. Growing prevalence of B-cell cancers, autoimmune disorders, and neurological conditions continues to drive demand for targeted treatments, and BTK inhibitors benefit from expanding indications and interest in safer, more precise therapies. At the same time, high development costs, uncertainty around long-term safety, and competition from other targeted and gene-based approaches create pressure on differentiation. In ICH, critical gaps in early diagnosis, monitoring, and effective interventions highlight both major opportunities and the scientific difficulty of improving outcomes. Altogether, the market shows solid potential, favoring companies that can deliver clear clinical benefits, strong evidence, and meaningful advances over existing options.
New York, USA, Dec. 17, 2025 (GLOBE NEWSWIRE) -- BTK Inhibitors Clinical Trial Pipeline Accelerates as 30+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
Overall, BTK inhibitors and ICH-related therapies sit in a space with strong scientific momentum but notable hurdles. Growing prevalence of B-cell cancers, autoimmune disorders, and neurological conditions continues to drive demand for targeted treatments, and BTK inhibitors benefit from expanding indications and interest in safer, more precise therapies. At the same time, high development costs, uncertainty around long-term safety, and competition from other targeted and gene-based approaches create pressure on differentiation. In ICH, critical gaps in early diagnosis, monitoring, and effective interventions highlight both major opportunities and the scientific difficulty of improving outcomes. Altogether, the market shows solid potential, favoring companies that can deliver clear clinical benefits, strong evidence, and meaningful advances over existing options.
DelveInsight’s 'BTK Inhibitors Pipeline Insight 2025' report provides comprehensive global coverage of pipeline therapies for BTK inhibitors across various stages of clinical development. The report offers an in-depth analysis of key trends, emerging therapies, and competitive landscape dynamics, highlighting the strategies of major pharmaceutical companies to advance the pipeline and capitalize on future growth opportunities. In addition, it includes critical insights into clinical trial benchmarking, partnering and licensing activities, and regulatory pathways involving the FDA and EMA, enabling stakeholders to make informed decisions and optimize development strategies within the BTK inhibitors domain.
BTK Inhibitors Clinical Trial Analysis Summary
- DelveInsight’s BTK inhibitors pipeline report depicts a robust space with 30+ active players working to develop 30+ pipeline BTK inhibitors.
- Key BTK inhibitor companies, such as Lupeng Pharmaceutical, ACEA Therapeutics, Roche , Merck KGaA , Biogen , HUTCHMED, TAIHO PHARMACEUTICAL CO., LTD., Hanmi Pharmaceutical, Evopoint Biosciences Co., Ltd., Hyperway Pharmaceutical Co.,Ltd., HealZen Therapeutics Co., Ltd., Crossfire Oncology and others, are evaluating new BTK inhibitor drugs to improve the treatment landscape.
- Promising pipeline BTK inhibitors such as Rocbrutinib, Abivertinib, Fenebrutinib, Nemtabrutinib, BIIB091, HMPL-760, TAS5315, Poseltinib, XNW1011, HBW-3210, HZ-A-018, CFON-026, and others are under different phases of BTK inhibitors clinical trials.
- Approximately 2+ BTK inhibitor drugs are in the late stage of development, whereas 10+ drugs are in the mid and early stages of development.
Request a sample and discover the recent advances in BTK inhibitor drugs @ BTK Inhibitors Pipeline Report
What are BTK Inhibitors?
Bruton tyrosine kinase (BTK) is a non-receptor tyrosine kinase that is essential for signal transduction through the B cell antigen receptor (BCR) and other surface receptors in both healthy and malignant B lymphocytes. Activation of BCR signaling in secondary lymphoid organs promotes the proliferation of malignant B cells, such as those seen in chronic lymphocytic leukemia (CLL). Over the past decade, BTK inhibitors (BTKi) have increasingly supplanted chemotherapy-based treatments, particularly for patients with CLL and mantle cell lymphoma (MCL). While BTKi show strong efficacy in CLL and MCL, they are also approved for use in Waldenström’s macroglobulinemia, small lymphocytic lymphoma, marginal zone lymphoma, and chronic graft-versus-host disease. However, long-term BTKi therapy is costly and may be associated with cumulative toxicities or resistance. Second-generation BTK inhibitors may offer a more favorable safety profile, supporting extended use, while combination therapies can achieve deeper remissions, potentially enabling limited-duration treatment.
Find out more about BTK inhibitor drugs @ BTK Inhibitors Analysis
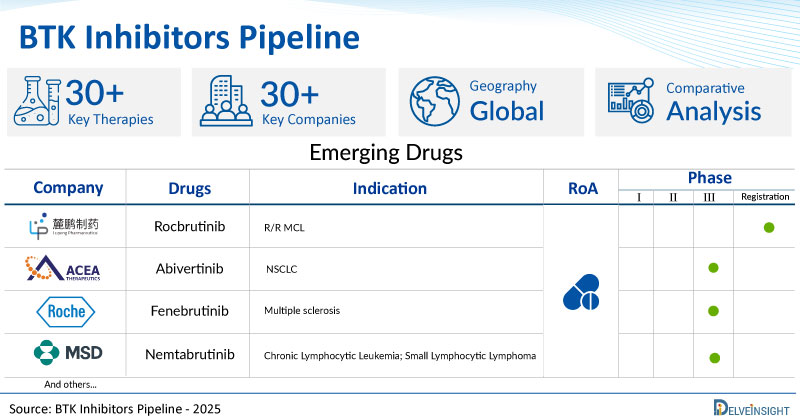
A snapshot of the Pipeline BTK Inhibitor Drugs mentioned in the report:
| Drugs | Company | Phase | Indication | RoA |
| Rocbrutinib | Lupeng Pharmaceutical | Registration | R/R MCL | Oral |
| Abivertinib | ACEA Therapeutics | III | NSCLC | Oral |
| Fenebrutinib | Roche | III | Multiple sclerosis | Oral |
| Nemtabrutinib | Merck KGaA Sharp & Dohme LLC | III | Chronic Lymphocytic Leukemia; Small Lymphocytic Lymphoma | Oral |
| BIIB091 | Biogen | II | Multiple sclerosis | Oral |
| HMPL-760 | HUTCHMED | II | Relapsed/Refractory Diffuse Large B-Cell Lymphoma | Oral |
| TAS5315 | Taiho Pharmaceutical | II | Rheumatoid arthritis | Oral |
| HBW-3210 | InnoCare Pharma | I/II | CNS Metastasis | Oral |
| Docirbrutinib | Carna Biosciences, Inc. | I | Hematological Malignancies | Oral |
Learn more about the emerging BTK inhibitors @ BTK Inhibitors Clinical Trials
Recent Developments in the BTK Inhibitor Treatment Space
- In November 2025, Roche announced that the first Phase III (FENhance 2) of two pivotal, similarly-designed Phase III studies (FENhance 1 and 2) in patients with relapsing multiple sclerosis (RMS) met its primary endpoint. Fenebrutinib, an investigational Bruton’s tyrosine kinase (BTK) inhibitor, significantly reduced the annualised relapse rate (ARR) compared to teriflunomide over a period of at least 96 weeks of treatment.
- In October 2025, Zenas BioPharma, Inc. and InnoCare Pharma Limited announced a transformational license agreement granting Zenas global development and commercialization rights to orelabrutinib for Multiple Sclerosis (MS) and across all therapeutic areas other than oncology. Orelabrutinib is a potentially best-in-class, highly selective CNS-penetrant, oral, small molecule BTK inhibitor with the potential to address compartmentalized inflammation and disease progression in MS.
- In August 2025, Dizal announced that the US Food and Drug Administration (FDA) has granted Fast Track Designation to its Birelentinib (DZD8586) for the treatment of adult patients with relapsed/refractory chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL) who have received at least two prior lines of therapy, including a BTK inhibitor and a BCL-2 inhibitor. Birelentinib is a first-in-class, non-covalent, LYN/BTK dual inhibitor with full blood-brain barrier (BBB) penetration.
- In July 2025, Everest Medicines announced updated positive results in the ongoing Phase Ib/IIa clinical trial for the treatment of primary membranous nephropathy (pMN) with EVER001 (previously known as XNW1011), a next-generation covalent reversible Bruton's tyrosine kinase (BTK) inhibitor with potentially best-in-class characteristics for the treatment of autoimmune renal diseases. Compared to covalent irreversible BTK inhibitors, EVER001 offers improved selectivity while maintaining high potency, thereby potentially avoiding many of the side effects associated with earlier-generation BTK inhibitors. Everest Medicines holds global rights to EVER001 for the treatment of renal diseases.
- In May 2025, the New Drug Application (NDA) for Rocbrutinib, the 4th generation Bruton's tyrosine kinase (BTK) inhibitor, independently developed by Lupeng Pharmaceutical, was formally accepted by the Center for Drug Evaluation (CDE) in China for the treatment of relapsed or refractory mantle cell lymphoma (R/R MCL) in adult patients who have previously received other BTK inhibitor therapies. If approved, Rocbrutinib is expected to become China’s first domestically developed BTK inhibitor used to treat this disease.
- In December 2024, Evopoint Biosciences announced that its partner Everest Medicines has reported positive results in Preliminary Analysis of Phase Ib/IIa Clinical Trial of EVER001 capsules (also known as XNW1011), a novel Bruton’s tyrosine kinase (BTK) inhibitor, for the treatment of primary membranous nephropathy (pMN). XNW1011 is a next-generation, covalent, reversible BTK inhibitor developed in-house by Evopoint Biosciences.
- In August 2024, Hansoh Pharmaceutical Group Co., Ltd. and Guangzhou Lupeng Pharmaceutical Co., Ltd. (Lupeng Pharma), jointly announced that the two parties have entered into a licensing agreement on a small molecule Bruton's tyrosine kinase inhibitor (BTKi) LP-168 (“LP-168”) independently developed by Lupeng Pharma.
- In June 2024, Hangzhou HealZen Therapeutics Co., Ltd announced that its independently developed Class 1 innovative drug, HZ-A-018 capsules (Bruton's tyrosine kinase inhibitor), has been officially approved by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA), China, to conduct a pivotal single-arm Phase II clinical study for the monotherapy of relapsed/refractory primary central nervous system lymphoma (R/R PCNSL).
Scope of the BTK Inhibitors Pipeline Report
- Coverage: Global
- BTK Inhibitors Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- BTK Inhibitors Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- BTK Inhibitors Therapeutics Assessment By Route of Administration: Oral, Parenteral, Intravenous, Subcutaneous, Topical
- BTK Inhibitors Therapeutics Assessment By Molecule Type: Monoclonal antibody, Peptides, Polymer, Small molecule, Gene therapy
- Key BTK Inhibitors Companies: Lupeng Pharmaceutical, ACEA Therapeutics, Roche , Merck KGaA , Biogen , HUTCHMED, TAIHO PHARMACEUTICAL CO., LTD., Hanmi Pharmaceutical, Evopoint Biosciences Co., Ltd., Hyperway Pharmaceutical Co.,Ltd., HealZen Therapeutics Co., Ltd., Crossfire Oncology, and others
- Key Pipeline BTK Inhibitors: Rocbrutinib, Abivertinib, Fenebrutinib, Nemtabrutinib, BIIB091, HMPL-760, TAS5315, Poseltinib, XNW1011, HBW-3210, HZ-A-018, CFON-026, and others
Dive deep into rich insights for new BTK inhibitors, visit @ BTK Inhibitors Drugs
Table of Contents
| 1. | BTK Inhibitors Pipeline Report Introduction |
| 2. | BTK Inhibitors Pipeline Report Executive Summary |
| 3. | BTK Inhibitors Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | BTK Inhibitors Clinical Trial Therapeutics |
| 6. | BTK Inhibitors Pipeline: Late-Stage Products (Pre-registration) |
| 7. | BTK Inhibitors Pipeline: Late-Stage Products (Phase III) |
| 8. | BTK Inhibitors Pipeline: Mid-Stage Products (Phase II) |
| 9. | BTK Inhibitors Pipeline: Early-Stage Products (Phase I) |
| 10. | BTK Inhibitors Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the BTK Inhibitors Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the BTK Inhibitors Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the BTK inhibitors pipeline therapeutics, reach out @ BTK Inhibitors Therapeutics
Related Reports
BTK Inhibitors Market Size, Target Population, Competitive Landscape & Market Forecast – 2034 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key BTK inhibitors companies, including BeiGene, Nurix Therapeutics, Roche , Genentech, Sanofi, Principia Pharma, Novartis , ACEA Therapeutics, InnoCare Pharma, among others.
Immune Thrombocytopenic Purpura Market
Immune Thrombocytopenic Purpura Market Insight, Epidemiology And Market Forecast – 2034 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key ITP companies, including Novartis , Takeda ( Millennium Pharmaceutical), Pfizer , Genosco, Oscotec, among others.
Non-small Cell Lung Cancer Market
Non-small Cell Lung Cancer Market Insight, Epidemiology And Market Forecast – 2034 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key NSCLC companies, including AstraZeneca, Boehringer Ingelheim, Pfizer , Takeda, Johnson & Johnson Innovative Medicine, Eli Lilly and Company, Merck KGaA , Bristol-Myers Squibb , Roche , Shanghai Henlius Biotech, AbbVie, Daiichi Sankyo, Nuvation Bio, PDC*line Pharma, Moderna Therapeutics, Pfizer , GSK, Gilead Sciences , BieGene, Nuvalent, among others.
Multiple Sclerosis Market Insight, Epidemiology And Market Forecast – 2034 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key multiple sclerosis companies, including Novartis , Sanofi, AB Science , Roche , Clene Nanomedicine, InnoCare Pharma, among others.
Follicular Lymphoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key follicular lymphoma Market companies, including Merck KGaA Sharp and Dohme, AstraZeneca, CRISPR Therapeutics, BeiGene, Nektar Therapeutics , NovalGen, Carna Biosciences, Allogene Therapeutics, Xynomic Pharmaceuticals, Bristol-Myers Squibb , Incyte Corporation, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com



