United States Lipid Nanoparticle Market Forecast and Competitive Analysis Report 2025-2033 Featuring Merck, Evonik, Genevant Sciences, CordenPharma, Arcturus, Ascendia, Acuitas, Croda
The United States lipid nanoparticle market is projected to expand from $210.34 billion in 2024 to $519.18 billion by 2033, with a CAGR of 10.56%. This growth is driven by the increasing use of lipid nanoparticles (LNP) in drug delivery, RNA-based therapies, and vaccines. Key factors include advancements in nanotechnology, increased pharmaceutical and biotech investments, and growing adoption of RNA therapeutics. Despite challenges like high production costs and regulatory complexities, strategic partnerships and technological innovations are enhancing LNP applications, particularly in precision medicine, oncology, and infectious diseases. The market is witnessing significant regional growth, with California, Texas, New York, and Florida leading the way. Prominent companies in the sector include Merck KGaA KGaA, Evonik Industries, and Genevant Sciences.
Dublin, Oct. 24, 2025 (GLOBE NEWSWIRE) -- The "United States Lipid Nanoparticle Market Report by Type, Application, End Use, States and Company Analysis, 2025-2033" report has been added to ResearchAndMarkets.com's offering.
United States Lipid Nanoparticle Market is expected to reach US$ 519.18 billion by 2033 from US$ 210.34 billion in 2024, with a CAGR of 10.56% from 2025 to 2033
The market for lipid nanoparticles in the US is anticipated to grow gradually due to its use in drug delivery, RNA-based treatments, and vaccinations, which are aided by developments in pharmaceutical research and nanotechnology. The market for lipid nanoparticles in the US is expanding regionally, with biotechnology hubs seeing the most activity. With the help of strong research institutes, biotech clusters, and rising healthcare investments, California, Texas, New York, and Florida are at the forefront of development. 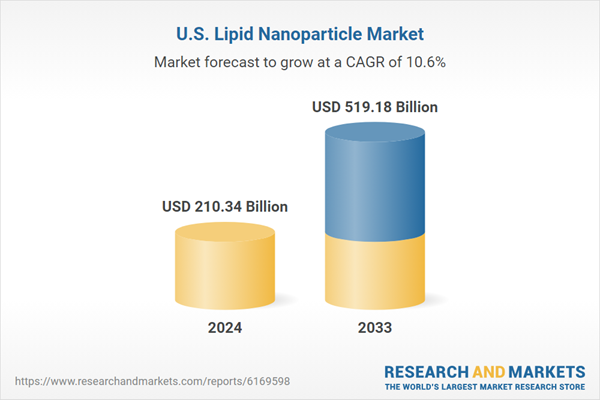
The lipid nanoparticle market in the US is expanding rapidly as nanotechnology continues to transform therapeutic applications and drug delivery. Because lipid nanoparticles (LNPs) can improve bioavailability, stability, and targeted administration, they are being used more and more as carriers for proteins, small compounds, and nucleic acids. LNPs have become a key technology in RNA-based treatments, such as gene therapy, vaccinations, and personalized medicine, in recent years. Research investments and partnerships between biotechnology businesses, pharmaceutical corporations, and academic institutions are being fueled by the growing need for safe and efficient drug delivery platforms. This developing field emphasizes the increasing use of lipid nanoparticles to treat unmet medical needs in infectious diseases, genetic disorders, and oncology.
Additionally, the need for LNP in the biotech and pharmaceutical sectors is being greatly impacted by the growing incidence of cancer worldwide. The American Cancer Society predicted that in 2024 alone, there will be 2,001,140 new cases of cancer in the United States and 611,720 deaths from the disease. The need for efficient and focused treatment alternatives is growing along with the incidence of cancer.
Advances in formulation methods, regulatory clearances, and the growing commercialization of RNA-based products all contribute to the market's growth. With the help of government-sponsored healthcare programs and venture capital funding, the US has emerged as a major center for LNP research and development. Lipid nanoparticles are being used by pharmaceutical companies to improve therapeutic efficacy and minimize side effects, and contract research organizations (CROs) are meeting demand by offering specialized services. The U.S. market is positioned as a leader in this industry due to the expansion of LNP applications brought about by the emergence of precision medicine and the continuous development of customized treatments.
High production costs, convoluted regulatory processes, and technological obstacles in large-scale manufacturing continue to be obstacles despite robust growth drivers. To get over these obstacles, businesses are spending more money on quality control systems and sophisticated production processes. The competitive landscape is also being shaped by strategic alliances and licensing contracts as businesses look to broaden their LNP portfolios and increase their worldwide presence. The U.S. lipid nanoparticle market is expected to continue its dynamic trajectory due to ongoing innovation and increased awareness of nanotechnology in healthcare, providing prospects for both well-established firms and up-and-coming biotech companies.
Key Factors Driving the United States Lipid Nanoparticle Market Growth
Growing Adoption in RNA-Based Therapeutics
The increasing use of RNA-based therapeutics, including mRNA vaccines and gene therapies, is one of the most significant growth drivers of the U.S. lipid nanoparticle market. LNPs serve as highly efficient delivery vehicles for nucleic acids, protecting them from degradation and ensuring effective transport into target cells. Their proven success in vaccine delivery has highlighted their potential for broader applications in treating rare genetic disorders, cancer, and chronic diseases.
Research pipelines across the country are increasingly incorporating lipid nanoparticle technologies to enhance drug efficacy and reduce side effects. This rising adoption is supported by both government funding initiatives and private sector investments, positioning lipid nanoparticles as essential to the future of modern medicine and driving continued market growth.
Advancements in Nanotechnology and Formulation Science
Continuous advancements in nanotechnology and formulation science are fueling the development of more effective lipid nanoparticles in the United States. Researchers are designing LNPs with improved stability, higher drug-loading capacity, and enhanced targeting capabilities, enabling better therapeutic outcomes. Innovations in lipid chemistry and production techniques are allowing companies to overcome challenges related to scalability and reproducibility, making these nanoparticles more commercially viable.
Academic collaborations with biotechnology firms are accelerating breakthroughs in precision delivery mechanisms, ensuring that therapeutic molecules reach their intended cellular destinations. The integration of artificial intelligence and computational modeling in nanoparticle design is also streamlining development processes. These technological strides are expanding LNP applications beyond vaccines, into oncology, regenerative medicine, and neurology, reinforcing their role as a driving force behind market expansion.
Rising Pharmaceutical and Biotech Investments
The surge in pharmaceutical and biotechnology investments is another critical driver of the U.S. lipid nanoparticle market. Leading pharmaceutical firms are actively incorporating LNP technologies into their pipelines to improve drug efficacy and safety. Venture capital firms and government programs are providing funding to startups and research institutions focused on nanoparticle innovations, creating a thriving ecosystem for development.
The success of LNP-based vaccines has demonstrated strong commercial potential, encouraging sustained financial backing. Strategic partnerships, acquisitions, and licensing agreements are further accelerating growth by expanding access to cutting-edge formulations and manufacturing capabilities. This influx of investment is not only supporting large-scale production but also encouraging the exploration of new therapeutic areas. As a result, the U.S. market continues to attract global attention, strengthening its position as an innovation hub.
Challenges in the United States Lipid Nanoparticle Market
High Production Costs and Scalability Issues
One of the major challenges facing the U.S. lipid nanoparticle market is the high cost associated with production and scalability. Manufacturing LNPs requires specialized facilities, advanced equipment, and highly skilled expertise to maintain consistency and quality. Scaling up from laboratory to commercial production often presents technical barriers, leading to increased costs and delays in market entry. Raw material shortages, particularly specialized lipids, add to pricing pressures and limit large-scale output.
Smaller biotech firms, in particular, struggle to balance research investment with manufacturing expenses, restricting their ability to compete with larger players. Although new technologies and process innovations are gradually reducing these challenges, production costs remain a critical hurdle. Overcoming these barriers is essential to ensure broader accessibility and adoption of LNP-based therapeutics in the United States.
Regulatory Complexity and Safety Concerns
Regulatory complexity and safety concerns represent another significant challenge for the U.S. lipid nanoparticle market. Since LNPs are relatively new in pharmaceutical applications, regulatory agencies require extensive safety data before granting approvals, resulting in lengthy and costly review processes. Uncertainties around long-term safety, potential immunogenicity, and off-target effects create additional obstacles for developers. Companies must navigate complex clinical trial designs and compliance standards, which often delay commercialization timelines.
For startups and smaller firms, these stringent requirements can act as major roadblocks to entry. Additionally, ongoing public debate regarding nanotechnology in healthcare adds pressure for transparent communication and stringent safety assessments. Addressing these regulatory and safety challenges will be vital for industry stakeholders to ensure market growth, foster public trust, and accelerate the development of innovative therapies.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 200 |
| Forecast Period | 2024 - 2033 |
| Estimated Market Value (USD) in 2024 | $210.34 Billion |
| Forecasted Market Value (USD) by 2033 | $519.18 Billion |
| Compound Annual Growth Rate | 10.5% |
| Regions Covered | United States |
Company Analysis: Overview, Key Persons, Recent Developments, SWOT Analysis, Revenue Analysis
- Merck KGaA KGaA
- Evonik Industries AG
- Genevant Sciences Corporation
- CordenPharma
- Arcturus Therapeutics, Inc.
- Ascendia Pharmaceuticals
- Acuitas Therapeutics
- Croda International Plc.
Market Segmentations
Type
- Liposomes
- Nanostructured Carriers
- Solid Lipid Nanoparticles
- Others
Application
- Therapeutics
- Research
End Use
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- Others
States
- California
- Texas
- New York
- Florida
- Illinois
- Pennsylvania
- Ohio
- Georgia
- New Jersey
- Washington
- North Carolina
- Massachusetts
- Virginia
- Michigan
- Maryland
- Colorado
- Tennessee
- Indiana
- Arizona
- Minnesota
- Wisconsin
- Missouri
- Connecticut
- South Carolina
- Oregon
- Louisiana
- Alabama
- Kentucky
- Rest of United States
For more information about this report visit https://www.researchandmarkets.com/r/vadvkz
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
CONTACT:
CONTACT: ResearchAndMarkets.com
Laura Wood, Senior Press Manager
press@researchandmarkets.com
For E.S.T Office Hours Call 1-917-300-0470
For U.S./ CAN Toll Free Call 1-800-526-8630
For GMT Office Hours Call +353-1-416-8900


