Hidradenitis Suppurativa Clinical Trial Analysis: Key Insights into Rich Pipeline Featuring 24+ Companies and 26+ Therapies | DelveInsight
The hidradenitis suppurativa market is poised for strong growth, driven by increasing disease awareness, earlier diagnosis, and expanding treatment access. Advances in biologic and targeted therapies, particularly the emergence of IL-17 and JAK inhibitors, are reshaping the therapeutic landscape and offering new hope for patients with limited options. Coupled with rising prevalence and significant unmet clinical needs, these factors position HS as an area of high potential for innovation and sustained market expansion.
New York, USA, Oct. 23, 2025 (GLOBE NEWSWIRE) -- Hidradenitis Suppurativa Clinical Trial Analysis: Key Insights into Rich Pipeline Featuring 24+ Companies and 26+ Therapies | DelveInsight
The hidradenitis suppurativa market is poised for strong growth, driven by increasing disease awareness, earlier diagnosis, and expanding treatment access. Advances in biologic and targeted therapies, particularly the emergence of IL-17 and JAK inhibitors, are reshaping the therapeutic landscape and offering new hope for patients with limited options. Coupled with rising prevalence and significant unmet clinical needs, these factors position HS as an area of high potential for innovation and sustained market expansion.
DelveInsight’s 'Hidradenitis Suppurativa Pipeline Insight 2025' report provides comprehensive global coverage of pipeline therapies for hidradenitis suppurativa across various stages of clinical development. The report offers an in-depth analysis of key trends, emerging therapies, and competitive landscape dynamics, highlighting the strategies of major pharmaceutical companies to advance the pipeline and capitalize on future growth opportunities. In addition, it includes critical insights into clinical trial benchmarking, partnering and licensing activities, and regulatory pathways involving the FDA and EMA, enabling stakeholders to make informed decisions and optimize development strategies within the hidradenitis suppurativa domain.
Hidradenitis Suppurativa Clinical Trial Analysis Summary
- DelveInsight’s hidradenitis suppurativa pipeline report depicts a robust space with 24+ active players working to develop 26+ pipeline hidradenitis suppurativa drugs.
- Key hidradenitis suppurativa companies such as InflaRx, MoonLake TX, Incyte Corporation, Avalo Therapeutics, Inc., Eli Lilly and Company, Sanofi, Merck KGaA Sharp & Dohme LLC, Almirall S.A., Insmed Incorporated, Citryll BV, Sonoma Biotherapeutics, Inc., Zura Bio, Shanghai Huaota Biopharmaceutical Co., Ltd., Leadingtac Pharmaceutical, AbbVie, Boehringer Ingelheim, and others are evaluating new hidradenitis suppurativa drugs to improve the treatment landscape.
- Promising pipeline hidradenitis suppurativa therapies, such as INF904, Sonelokimab, INCB54707, AVTX 009, LY3041658, Brivekimig, Tulisokibart, LAD191, Brensocatib, CIT-013, SBT777101, Tibulizumab, HB0043, LT 002 158, Upadacitinib, Spesolimab, and others, are in different phases of hidradenitis suppurativa clinical trials.
- Approximately 4+ hidradenitis suppurativa drugs are in the late stage of development, whereas 20+ drugs are in the mid and early stages of development.
- Notable MoAs in hidradenitis suppurativa clinical trials include Janus kinase 1 inhibitors, IL17A protein inhibitors, IL17F protein inhibitors, Chemokine inhibitors, Complement C5a receptor antagonists, IRAK4 degraders, Interleukin 36 receptor antagonists, Regulatory T-lymphocyte replacements, and others.
Request a sample and discover the recent advances in hidradenitis suppurativa medication @ Hidradenitis Suppurativa Pipeline Report
What is Hidradenitis Suppurativa?
Hidradenitis suppurativa, also known as acne inversus, is a long-lasting inflammatory skin disorder characterized by deep nodules, abscesses, draining tunnels, and fibrotic scars. These lesions typically develop in intertriginous regions and areas with a high concentration of apocrine glands, with the axillae, groin, perianal, perineal, and inframammary areas being most commonly affected. This activity explores the causes, clinical features, and complications of HS, emphasizing the importance of an interprofessional approach to management. The disease process starts when a defective hair follicle becomes blocked and ruptures, releasing keratin, bacteria, and other contents into the surrounding dermis. This triggers a chemotactic inflammatory response from neutrophils and lymphocytes, which can lead to abscess formation and damage to the pilosebaceous unit and surrounding tissues. The prognosis varies among patients, and no definitive cure has been found. Outcomes are poorer when diagnosis and treatment are delayed, particularly in the early stages, and when comorbidities such as smoking and obesity are not addressed.
Find out more about biologics approved for hidradenitis suppurativa treatment @ FDA Approved Biologics For Hidradenitis Suppurativa
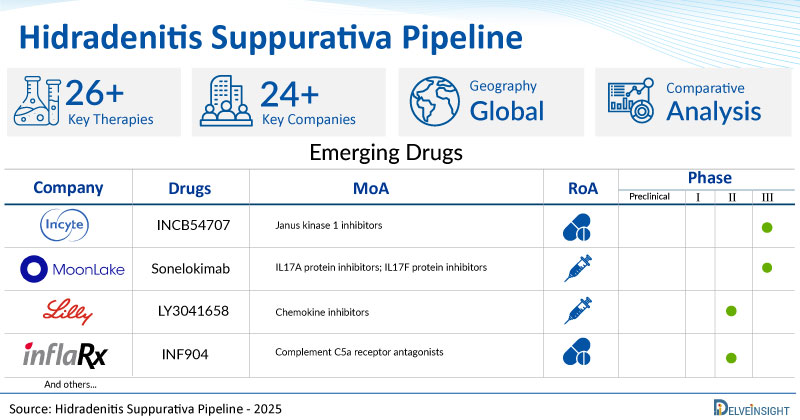
A snapshot of the Pipeline Hidradenitis Suppurativa Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| INCB54707 | Incyte Corporation | III | Janus kinase 1 inhibitors | Oral |
| Sonelokimab | MoonLake Immunotherapeutics | III | IL17A protein inhibitors; IL17F protein inhibitors | Subcutaneous |
| LY3041658 | Eli Lilly and Company | II | Chemokine inhibitors | Subcutaneous |
| INF904 | InflaRx | II | Complement C5a receptor antagonists | Oral |
| LT 002 158 | Leadingtac Pharmaceutical | I/II | IRAK4 degraders | Oral |
| HB0043 | Shanghai Huaota Biopharmaceutical Co., Ltd. | I/II | IL17A protein inhibitors; Interleukin 36 receptor antagonists | Intravenous |
| SBT 77 7101 | Sonoma Biotherapeutics, Inc. | I | Regulatory T-lymphocyte replacements | Parenteral |
Learn more about the new medication for hidradenitis suppurativa treatment @ New Med for Hidradenitis Suppurativa
Recent Developments in Hidradenitis Suppurativa Treatment Space
- In September 2025, MoonLake Immunotherapeutics announced the week 16 results of the Phase III VELA-1 and VELA-2 trials of its registrational global program for Sonelokimab in patients with moderate-to-severe hidradenitis suppurativa (HS).
- In September 2025, Sanofi announced new data from the HS-OBTAIN phase IIa study, showing that treatment with brivekimig led to clinically meaningful improvements in the primary endpoint of Hidradenitis Suppurativa Clinical Response (HiSCR50) in patients naïve to biologics with moderate-to-severe hidradenitis suppurativa (HS). Brivekimig was well tolerated, with no serious adverse events. The results will be shared in an oral presentation at the European Academy of Dermatology and Venereology (EADV) 2025 Congress in Paris.
- In May 2025, Zura Bio Limited announced the launch of TibuSHIELD, a global Phase II clinical study evaluating tibulizumab in adults with moderate to severe HS. TibuSHIELD is designed to enroll approximately 180 adults with moderate to severe HS across the United States, Canada, and Europe. The study will evaluate tibulizumab over a 28-week period, comprising a 16-week primary efficacy assessment and a 12-week safety follow-up, with an optional OLE.
- In March 2025, Incyte announced positive topline results from its pivotal Phase III STOP-HS clinical trial program evaluating the safety and efficacy of povorcitinib, an oral small-molecule JAK1 inhibitor, in adult patients (≥18 years) with moderate to severe hidradenitis suppurativa (HS).
- In December 2024, Ikena Oncology, Inc. and Inmagene Biopharmaceuticals announced they have entered into a definitive merger agreement. In connection with the merger, Ikena has entered into subscription agreements for a USD 75 million private placement. The combined company will focus on the development of IMG-007, a monoclonal antibody (mAb) targeting OX40. The combined company plans to operate under the name “ImageneBio, Inc.” and trade on NASDAQ under the ticker “IMA”. IMG-007 is a mAb targeting OX40 with potential utility in a wide range of inflammatory indications, including atopic dermatitis, asthma, hidradenitis suppurativa, systemic sclerosis and others.
- In July 2024, Avalo Therapeutics, Inc. announced that the Investigational New Drug (IND) for AVTX-009, an anti-IL-1β monoclonal antibody (mAb), for the treatment of hidradenitis suppurativa (HS) is now active, permitting the Company to commence its Phase II (LOTUS) clinical trial in patients with HS.
Scope of the Hidradenitis Suppurativa Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Parenteral, Intravenous, Subcutaneous, Topical
- Therapeutics Assessment By Molecule Type: Monoclonal antibody, Peptides, Polymer, Small molecule, Gene therapy
- Therapeutics Assessment By Mechanism of Action : Janus kinase 1 inhibitors, IL17A protein inhibitors; IL17F protein inhibitors, Chemokine inhibitors, Complement C5a receptor antagonists, IRAK4 degraders, Interleukin 36 receptor antagonists, Regulatory T-lymphocyte replacements
- Key Hidradenitis Suppurativa Companies: InflaRx, MoonLake TX, Incyte Corporation, Avalo Therapeutics, Inc., Eli Lilly and Company, Sanofi, Merck KGaA Sharp & Dohme LLC, Almirall S.A., Insmed Incorporated, Citryll BV, Sonoma Biotherapeutics, Inc., Zura Bio, Shanghai Huaota Biopharmaceutical Co., Ltd., Leadingtac Pharmaceutical, AbbVie, Boehringer Ingelheim, and others.
- Key Hidradenitis Suppurativa Pipeline Therapies: INF904, Sonelokimab, INCB54707, AVTX 009, LY3041658, Brivekimig, Tulisokibart, LAD191, Brensocatib, CIT-013, SBT777101, Tibulizumab, HB0043, LT 002 158, Upadacitinib, Spesolimab, and others.
Dive deep into rich insights for new hidradenitis suppurativa treatments, visit @ Hidradenitis Suppurativa Drugs
Table of Contents
| 1. | Hidradenitis Suppurativa Pipeline Report Introduction |
| 2. | Hidradenitis Suppurativa Pipeline Report Executive Summary |
| 3. | Hidradenitis Suppurativa Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Hidradenitis Suppurativa Clinical Trial Therapeutics |
| 6. | Hidradenitis Suppurativa Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Hidradenitis Suppurativa Pipeline: Late-Stage Products (Phase III) |
| 8. | Hidradenitis Suppurativa Pipeline: Mid-Stage Products (Phase II) |
| 9. | Hidradenitis Suppurativa Pipeline: Early-Stage Products (Phase I) |
| 10. | Hidradenitis Suppurativa Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Hidradenitis Suppurativa Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Hidradenitis Suppurativa Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the hidradenitis suppurativa research 2025, reach out @ Hidradenitis Suppurativa New Treatment 2025
Related Reports
Hidradenitis Suppurativa Epidemiology Forecast
Hidradenitis Suppurativa Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted CHF epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Hidradenitis Suppurativa Market
Hidradenitis Suppurativa Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Hidradenitis Suppurativa companies, including Incyte Corporation, AbbVie, MoonLake Immunotherapeutics, Boehringer Ingelheim, Priovant Therapeutics, Eli Lilly, Sanofi, Kymera Therapeutics, UNION Therapeutics, among others.
Atopic Dermatitis Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key atopic dermatitis companies including Arcutis Biotherapeutics, Amgen , Kyowa Kirin, Dermavant Sciences, Cara Therapeutics, Pfizer , Arena Pharmaceuticals , BioMimetix, Eli Lilly and Company, Aldeyra Therapeutics , Inc., Hangzhou Yirui Pharmaceutical Technology Co., Ltd, LEO Pharma, Corvus Pharmaceuticals, Inc., Brexogen Inc., Sanofi, Shaperon, UCB Pharma, Q32 Bio Inc., Akeso, Apogee Therapeutics, Inc., Allakos Inc ., Biosion, Inc., among others.
Atopic Dermatitis Clinical Trial Analysis
Atopic Dermatitis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key atopic dermatitis companies, including Kymab, BiomX, LEO Pharma, GlaxoSmithKline , Arjil Pharmaceuticals, SCM Lifescience, Sun Pharmaceutical Industries Limited, Brickell Biotech Inc, Dermira, AstraZeneca, Kyowa Kirin, UCB Biopharma, Arcutis Biotherapeutics, and others. Kymab, BiomX, LEO Pharma, GlaxoSmithKline , Arjil Pharmaceuticals, SCM Lifescience, Sun Pharmaceutical Industries Limited, Brickell Biotech Inc, AstraZeneca, Kyowa Kirin, UCB Biopharma, Arcutis Biotherapeutics, Vanda Pharmaceuticals, Kyowa Kirin, Sanofi, KeyMed Biosciences, Asana BioSciences, Bristol-Myers Squibb , RAPT Therapeutics, Allakos, Novartis , BioMimetix, Shanghai Hengrui Pharmaceutical Co, Connect Group Biopharma, Pfizer , Evommune, Inc., Fresh Tracks Therapeutics, Biosion, Chia Tai Tianqing Pharmaceutical, Reistone Biopharma Company Limited, JW Pharmaceutical, Oneness Biotech, Alphyn Biologics, selectION, UNION Therapeutics, Ichnos Scien, among others.
Psoriasis Clinical Trial Analysis
Psoriasis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key psoriasis companies, including Mylan , Biocad, Bristol-Myers Squibb , Celltrion, Coherus BioSciences, Janssen Pharmaceuticals, Can-Fite Biopharma, Arcutis Biotherapeutics, Amgen , Iltoo Pharma, GlaxoSmithKline , Galectin Therapeutics, Evelo Biosciences, Galderma, BioMimetix JV, Menlo Therapeutics Inc ., Aristea Therapeutics, UNION Therapeutics, MetrioPharm, Sienna Biopharmaceuticals, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect Group with us at LinkedIn
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com



